Thermodynamics is a branch of physics deals with heat and temperature and their relation to energy and work. It is macroscopic variables such as internal energy, entropy, and pressure, that describe a body of matter or radiation. It states that the behavior of those variables is subject to general constraints, that are common to all materials, not the peculiar properties of particular materials. These general constraints are expressed in the four laws of thermodynamics. Thermodynamics describes the bulk behavior of the body, not the microscopic behaviors of the very large numbers of its microscopic constituents, such as molecules.
Firstly thermodynamics applied to heat engines, was concerned with the thermal properties of their materials working such as steam, to increase the efficiency and power output of engines.
Equation:
There are Four principal laws of thermodynamics which are described on separate. Each law is
the definition of thermodynamic properties which help us to understand and predict the operation of a physical system.
Zeroth Law of Thermodynamics
First Law of Thermodynamics
Second Law of Thermodynamics
Third Law of Thermodynamics
Examples:
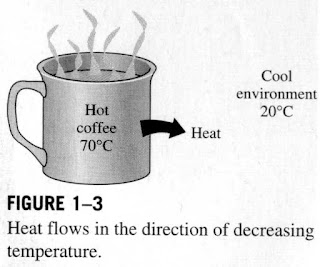
1- Heat is energy converted from one form to another, or transferred from one object to another. For example, a stove burner converts electrical energy to heat and conducts energy through the pot to the water. This increases the kinetic energy of the water molecules, causing them to move faster and faster. At a certain temperature the boiling point, the atoms have gained enough energy to break free of the molecular bonds of the liquid and escape as vapor.
2- Minimizing heat loss in winter and heat gain in summer. The size and location and the power input of the fan of your computer is also selected after an analysis that involves thermodynamics.
3- Conservation of energy for the human body is also an example of thermodynamics.
Application of Thermodynamics: